FDA Approves Roche and BD’s New Self-Collection HPV Tests for Enhanced Cervical Cancer Screening in the U.S.
Fiona Nanna, ForeMedia News
8 minutes read. Updated 7:08AM GMT Thurs, 5 September, 2024
Patients in the United States now have a new option for their gynecological visits. The U.S. Food and Drug Administration (FDA) recently approved self-collection HPV tests, allowing individuals to collect their own vaginal samples for cervical cancer screening rather than undergoing traditional Pap smears. This change aims to make screenings more accessible and less intimidating, potentially improving early detection rates.
The Shift in Screening Protocols
The introduction of self-collection HPV tests marks a considerable evolution in cervical cancer screening. Traditionally, Pap smears require a speculum to collect cells from the cervix for examination, a process that can be uncomfortable for many patients. With self-collection, patients can now provide their own samples, similar to how they might collect urine samples, but within a healthcare setting such as a doctor’s office, urgent care, or pharmacy clinic.
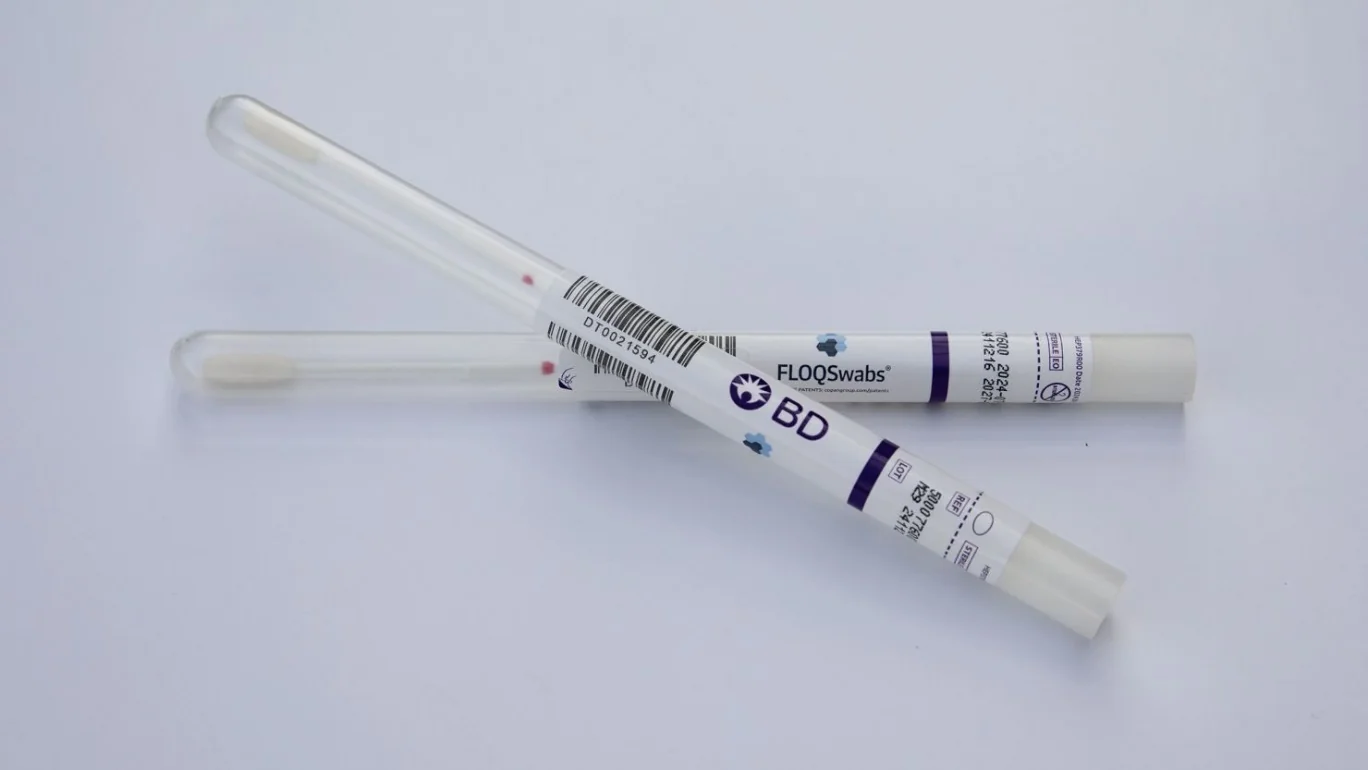
Two major players in the healthcare industry, Roche and BD, have already begun distributing these self-collection tests. Roche expects its HPV self-collection screening solution to be available this fall, while BD has started shipping its tests as of last Thursday. These tests, designed to detect high-risk types of HPV that are known to cause cervical cancer, are anticipated to enhance screening accessibility, particularly for those who may have previously avoided the procedure.
Statistics and Impact
Cervical cancer remains a serious health concern, with more than 11,000 new cases diagnosed annually in the United States, according to the Centers for Disease Control and Prevention (CDC). Approximately 4,000 women die from the disease each year. Data suggests that about half of invasive cervical cancer cases are found in individuals who have never been screened, and 10% occur in those who have not been screened in the past five years.
Dr. Jeff Andrews, a board-certified gynecologist and Vice President of Global Medical Affairs for Diagnostic Solutions at BD, emphasizes the potential benefits of self-collection. “The goal is to reach women who are under-screened by providing a method that might appeal more to those who might otherwise skip their screenings,” he explains.
Global Perspective
Countries in Europe, Australia, and New Zealand have already implemented self-collection HPV testing with positive results. The hope is that this method will similarly encourage higher screening rates in the United States, where approximately 30% of women are either unscreened or under-screened. “By offering an alternative, we can reduce the incidence of cervical cancer,” notes Dr. John Vullo, Chairman of Obstetrics and Gynecology at Catholic Health’s Good Samaritan University Hospital. “It’s essential to provide more options to improve accessibility for underserved populations.”
Screening Methods and Future Possibilities
Current guidelines from the U.S. Preventive Services Task Force recommend cervical cancer screening every three years for women aged 21 to 29, and every three to five years for women aged 30 to 65, depending on the type of test used. Self-collection HPV tests are designed to complement these existing protocols by offering a less invasive option.
While self-collection is currently available only in healthcare settings, companies like BD and Teal Health are working towards making these tests available for home use. Teal Health’s Teal Wand, granted “breakthrough device” status by the FDA, represents a promising step towards at-home testing.
Cervical cancer screening remains a crucial aspect of women’s health. The advent of self-collection HPV tests represents a significant step forward in making these screenings more comfortable and accessible. As the healthcare community adapts to this new option, it is hoped that more individuals will take advantage of screening opportunities, ultimately leading to better prevention and early detection of cervical cancer.
Meta Description: Discover how new self-collection HPV tests are changing cervical cancer screening in the U.S., offering a more accessible and comfortable alternative to traditional Pap smears. Learn about the potential benefits and future of self-collected tests.