Experts Call Twice-Yearly HIV Injection Offering 100% Protection a ‘Game-Changer’ for AIDS Prevention
Fiona Nanna, ForeMedia News
7 minutes read. Updated 1:29PM GMT Fri, 26July, 2024
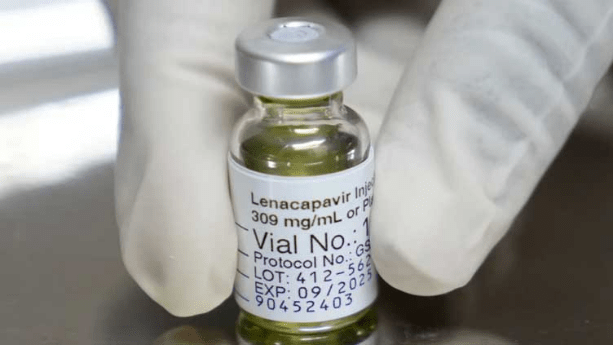
In a groundbreaking study recently published in the New England Journal of Medicine and discussed at the AIDS conference in Munich, researchers have unveiled promising results for a twice-yearly injection designed to prevent HIV infection. The injection, produced by U.S. pharmaceutical company Gilead and marketed as Sunlenca, demonstrated a remarkable 100% efficacy in preventing new HIV infections among women. This revelation is being described by experts as nothing short of “stunning.”
Breakthrough Results
The study, conducted with approximately 5,000 participants across South Africa and Uganda, showed that none of the young women and girls receiving the Sunlenca injections contracted HIV. In contrast, among those who took daily prevention pills, around 2% were infected by HIV from their partners. Salim Abdool Karim, a prominent AIDS researcher from Durban, South Africa, expressed astonishment at the study’s results, calling the level of protection “stunning.”
Sunlenca’s Current Status and Future Prospects
While Sunlenca is already approved in the U.S., Canada, Europe, and other regions as a treatment for HIV, it has yet to be authorized for prevention. Gilead is awaiting results from ongoing studies in men before seeking regulatory approval for preventive use. The promising results from the current study led to its early termination, allowing all participants to receive the injections. Sunlenca, also known as lenacapavir, is anticipated to revolutionize HIV prevention efforts due to its biannual administration, which significantly contrasts with the daily pill regimen.
Challenges and Concerns
Despite the optimism surrounding Sunlenca, there are concerns about its affordability. Gilead has not yet established a price point for the drug in low-income countries, where the need for affordable HIV prevention is most critical. The company has indicated plans to implement a “voluntary licensing program,” which would permit a limited number of generic producers to manufacture the drug. Winnie Byanyima, Executive Director of the U.N. AIDS agency, has urged Gilead to share Sunlenca’s patent through a U.N.-backed program to facilitate access in poorer nations.
Dr. Helen Bygrave of Doctors Without Borders emphasized that if priced affordably, Sunlenca could be a game-changer in reversing the HIV epidemic, particularly in countries with high infection rates. She urged Gilead to provide a more accessible price for the drug.
The Broader Context
In light of Sunlenca’s potential, the global health community is eagerly awaiting further developments. UNAIDS reports a decline in new HIV infections globally, yet challenges persist, especially in regions such as Eastern Europe, Latin America, and the Middle East. An alternative HIV prevention shot, Apretude, is available in some countries but remains too expensive for widespread use.
Olwethu Kemele, a health worker at the Desmond Tutu Health Foundation in South Africa, predicts that the new injection could significantly increase the uptake of HIV prevention services. The ease of a biannual injection could address issues of adherence and stigma associated with daily pills.
The recent study on Sunlenca represents a monumental step forward in HIV prevention. With potential to transform the landscape of AIDS prevention, it echoes the historic shift in HIV treatment witnessed decades ago when access to antiretroviral drugs was expanded. As Gilead moves toward scaling up production and determining a fair price, the global health community remains hopeful that this innovation will soon become a cornerstone of HIV prevention strategies worldwide.